
Shino Manabe
Professor, School of Pharmacy and Pharmaceutical Sciences, Hoshi University & Research Center for Pharmaceutical Development Graduate School of Pharmaceutical Sciences & Faculty of Pharmaceutical Sciences, Tohoku University
She received her Ph.D. from the Graduate School of Pharmaceutical Sciences at the University of Tokyo in 1996. She began her academic career as a Teaching Assistant in the same department in April of that year. In October 1996, she joined RIKEN as a Special Postdoctoral Researcher and was later appointed as a Research Scientist in 2000. In 2019, she assumed a cross-appointed professorship at the Center for Drug Development, Graduate School of Pharmaceutical Sciences, Tohoku University. Since 2020, she has been serving as a Professor at the Faculty of Pharmaceutical Sciences, Hoshi University.
Her research centers on the development of stereoselective glycosylation reactions and the chemical synthesis of biologically active glycans and glycoconjugates, with a strong foundation in organic chemistry.
Recently, the market for antibody therapeutics has expanded, and glyco-engineering has emerged as a technology that enhances antibody functions. Specifically, controlling the N-linked glycan structure in the Fc region of IgG can enhance antibody-dependent cellular cytotoxicity (ADCC) and optimize pharmacokinetics. Conventional glycan structure analysis faced challenges in retaining positional and combinatorial data. However, recent advances in glycan homogenization using endoglycosidases mutants have enabled more precise glyco-engineering techniques. Additionally, this technology has been applied to the development of homogeneous antibody-drug conjugates (ADCs), offering a promising strategy for cancer treatment. This review provides an overview of recent advances in IgG glycoengineering and its potential applications in drug development.
Recently, antibody therapeutics have steadily increased their share in the pharmaceutical market. IgG universally carries a pair of N-linked glycans attached to the Asn297 residue in the Fc region. By precisely controlling and modifying the structure of these glycans, antibody function can be enhanced, enabling the development of novel therapeutics. A representative example is the removal of core fucose that increases ADCC by 50 to 100 times1. This technology has become the foundation for highly successful glycoengineered antibodies in clinical use. Additionally, the glycan structures of IgG affect its pharmacokinetics and thermal stability. Moreover, antibody-producing cell-derived non-human glycan structures, such as N-glycolylneuraminic acid and Galα1,3-Gal, have been reported to exhibit immunogenicity2.
Owing to their multistep and complex biosynthetic pathways, N-linked glycans exist as heterogeneous structural compounds. This characteristic has been observed even in therapeutic antibodies, where the proportion of glycan structures has been shown to vary across production batches3. Traditional glycan structural analysis relies on cleaving glycans from glycoproteins, resulting in the loss of data regarding glycan attachment sites and structural combinations. The absence of these crucial data hinders the precise determination of glycoprotein structures and poses a significant challenge in establishing clear associations between glycan structures and antibody function.
To address this challenge, a glycan homogenization method for IgG was developed4. This process involves the following steps: first, the heterogeneous glycans are removed using an endo-type glycosidase—endo-β-N-acetylglucosaminidase (ENGase)—that cleaves the bond between chitobiose at reducing end, leaving only the N-acetylglucosamine at the reducing terminus. This process eliminates the heterogeneous non-reducing terminal portion and immunogenic components. To date, numerous ENGases have been isolated. However, for IgG glycan modification, EndoS, Endo S2, and their mutants are commonly used5. Subsequently, a homogeneous glycan structure was introduced into the deglycosylated IgG using a ENGase mutant that retained its glycan transfer activity while suppressing its original hydrolytic activity. Oxazoline glycan, an analog of the hydrolysis intermediate, was used as a glycan donor. Additionally, for industrial applications, an automated process using flow synthesis technology has been reported for this antibody modification6. Moreover, this glycan modification technology has been applied to the development of homogeneous antibody-drug conjugates, as discussed later.
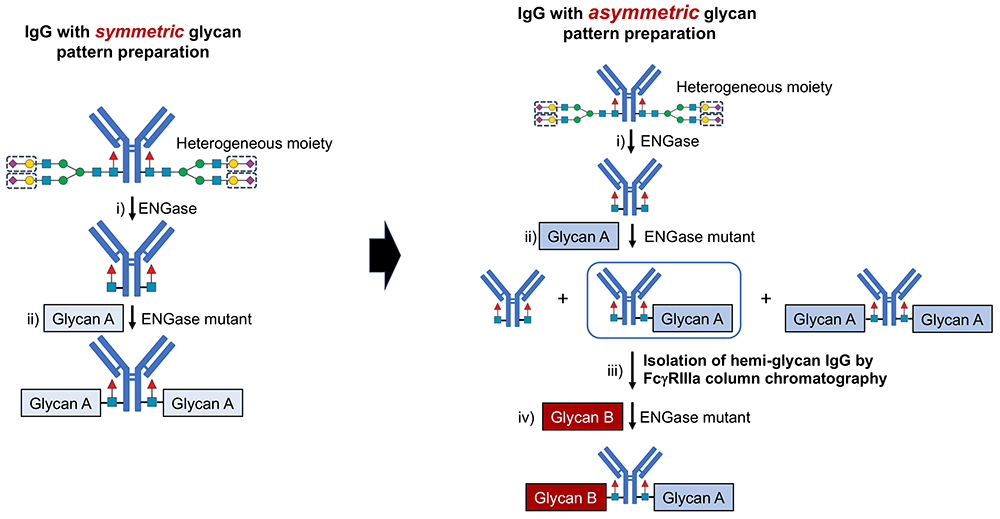
Conventional methods for producing glycan-homogeneous IgGs are limited to generating antibodies with identical glycan pairs (symmetric type). However, in natural IgGs, the majority of antibodies have different glycans in each pair (asymmetric type). Developing a method to produce asymmetric glycan-homogeneous IgG remains unexplored.
FcγRIIIa affinity chromatography analysis revealed that IgG retention time varies significantly based on the number of glycans. The ADCC of IgG is mediated by FcγRIIIa, which activates effector cells upon antibody binding, resulting in the destruction of target cells through the release of cytokines, perforin, granzymes, and other granule contents. FcγRIIIa affinity chromatography—that is closely associated with ADCC—was developed as a column capable of measuring the ADCC potential of IgG7.
Based on this finding, we developed a method using FcγRIIIa affinity chromatography to isolate hemi-glycosylated IgG and add different glycan structures to produce asymmetric glycan-homogeneous IgG. This approach successfully enabled us to create a library of 66 glycan-homogeneous variants of trastuzumab—a widely used antibody therapeutic8.
When the ADCC of the glycan-homogeneous trastuzumab variants was measured, we identified one symmetric and ten asymmetric glycan structures that exhibited high ADCC. Additionally, IgG with two galactose residues (G2) exhibited stronger ADCC activity than those with zero (G0) or one galactose residue (G1a-F and G1b-F). Notably, even with the same number of galactose residues, G1a-F (containing a Manα1-6 branch) exhibited higher ADCC activity than that of G1b-F (containing a Manα1-3 branch). Similarly, among structures with similar numbers of sialic acid and galactose residues, A1a-F (containing a Manα1-6 branch) exhibited higher ADCC activity than that of A1b-F (containing a Manα1-3 branch).
Additionally, differential scanning calorimetry (DSC) was performed to assess the thermal stability of N-acetylneuraminic acid. The results revealed a proportional relationship between the melting temperature (Tm) and enthalpy (ΔH) change in the A1b, A1b-Gal, and M3 series. This phenomenon cannot be elucidated using conventional methods that involve the extraction of glycans from proteins for analysis. These findings highlight the advantages of measuring intact glycoproteins and provide valuable insights into glycan behavior.
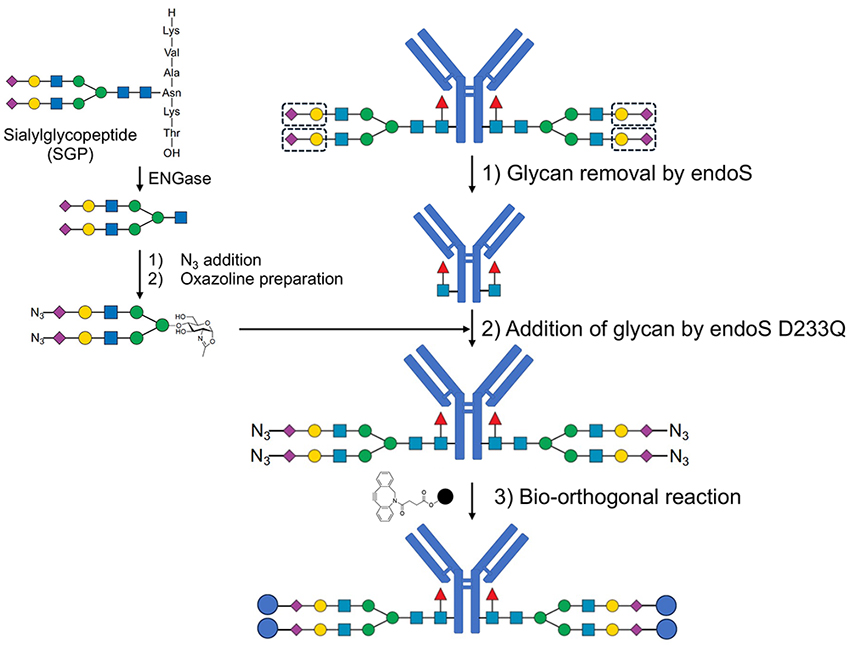
Recent advancements in antibody glycan modification technology have enabled the production of ADCs with homogeneous structures. ADCs are formed by linking highly cytotoxic drugs to antibodies through linkers and are gaining attention as promising therapeutic modalities for cancer treatment. The therapeutic mechanism of ADCs begins with their penetration into the tumor tissue through tumor-associated blood vessels via the enhanced permeability and retention (EPR) effect9. Subsequently, ADCs bind to the surface of target antigen-expressing cancer cells, followed by cellular internalization. Once inside, the drug is primarily released in lysosomes by specific enzymes, resulting in the destruction of the target cells. ADCs are a highly precise form of chemotherapy based on the concept of a drug delivery system.
Currently, ADCs use random-conjugation, attaching drugs through cysteine or lysine residues. However, this approach lacks precise control over the number of payloads per antibody or their attachment sites, resulting in structural heterogeneity. Studies have reported that differences in payload attachment sites affect ADC stability and pharmacokinetics. Therefore, the production of ADCs with homogeneous structures is crucial for expanding their therapeutic window. Additionally, ADC mixtures produced through random-conjugation exhibit low reproducibility during manufacturing and pose challenges in quality control. To address these issues, site-specific conjugation technologies that selectively attach drugs to specific sites on the antibodies are being actively developed. This technology enables the stable production of ADCs with homogeneous structures, thereby enhancing therapeutic efficacy and manufacturing consistency.
Antibody glycan modification technology enables the production of structurally homogeneous ADCs. Attempts to create homogeneous ADCs have been reported using methods, such as in vitro protein synthesis and enzyme-mediated approaches, to introduce bio-orthogonal functional groups, such as azides. In this study, we used glycan modification technology to conjugate payloads specifically to glycan sites, thereby producing structurally homogeneous ADCs10. First, an azide group was introduced into the carboxyl group of N-acetylneuraminic acid in N-linked glycans derived from egg yolk through an amide bond formation, followed by oxazoline modification at the reducing terminus11. Subsequently, trastuzumab was deglycosylated using EndoS, and the modified glycan containing an azide group was reattached using a mutant form of EndoS (D233Q), resulting in an antibody with a homogeneous glycan structure. Finally, a payload containing a strained alkyne group is conjugated to the antibody through a bio-orthogonal reaction, thereby successfully constructing a structurally homogeneous ADC.
Biological activity assessments of the resulting ADC demonstrated highly cytotoxic effects against human epidermal growth factor receptor 2 (HER2)-overexpressing cell lines (SK-BR3, OE-19, and N-87), while exhibiting minimal activity against HER2-low-expressing cell lines (MKN-45 and MCF-7). Additionally, owing to the uniformity of the glycan structure, we confirmed that ADCC of ADC analogue via N-glycan linkage can be controlled through FcγRIIIa column chromatography analysis12. Further investigations are underway to assess the in vivo antitumor effects of ADC.
Using antibody glycan modification technology, we assessed IgG production with a homogeneous glycan structure and its potential applications in pharmaceutical development. Specifically, we demonstrated that glycan regulation can enhance ADCC and optimize pharmacokinetics, highlighting the use of glycan homogenization technology. Additionally, we applied this technology to produce structurally homogeneous ADCs and confirmed their selective cytotoxic effects on the target cells. Further advancements in glycan modification technology are anticipated to drive the development of highly functional biopharmaceuticals, paving the way for novel therapeutic strategies, including cancer treatment.