
Yoshihiko Murakami
Professor, Department of Applied Chemistry, Faculty of Engineering, Tokyo University of Agriculture and Technology
He received his Ph.D. from the Graduate School of Engineering at Waseda University in 2000. He has worked at Waseda University (2000-2001), RIKEN (Bioengineering Laboratory, 2001-2004), and KAST (Polymeric nanomedical project, 2004-2006). He came to Tokyo University of Agriculture and Technology in 2006 and became a professor in 2019. He received several awards, including the SCEJ Award for Outstanding Young Researcher (The Society of Chemical Engineers, Japan) in 2005, the Award for Young Investigator of Japanese Society for Biomaterials (Japanese Society for Biomaterials) in 2012, and the Oleo Science Award (Japan Oil Chemists’ Society) in 2020.
Gels, three-dimensional structures formed by polymers, have various biosimilar properties such as water retention, structural flexibility, and material retention, and are being considered for a wide range of medical applications including as materials in soft contact lenses, artificial muscles, artificial breasts, artificial skin, and as anti-adhesive materials, wound healing materials, and drug carriers in drug delivery systems. This article focuses on how polysaccharide gels and gel-sheets with both tissue adhesion and drug release properties are prepared by combining polysaccharides (hyaluronic acid and chitosan) and synthetic polymers.
Gels that adhere to living tissues (i.e., tissue-adhesive materials) are used to assist adhesion, for example, by applying them to the suture line after an artificial blood vessel is connected to an existing living blood vessel or an artificial blood vessel is connected to the heart. They can also be used to prevent adhesion by acting as a barrier, when applied, to tissue surface adherence to other surfaces. In recent years, their application as hemostatic agents has also been attracting attention, especially as hemostatic materials for stopping extravasation of blood (slow leakage of blood from sources that are not immediately apparent). If this tissue-adhesive material can be used to sustain drug release, it will be possible to create a drug-releasing material that is implantable by subcutaneous injection of a liquid gel-precursor substance into the body and subsequent gelation inside the body.
Although various tissue-adhesive gels have been developed, further development efforts are needed to achieve gels with properties that meet medical practice requirements, including high biocompatibility, rapid gelation, and strength suitable for the site of application. In addition, when gels are applied to the living body, the therapeutic effect may be enhanced by the combination use of drugs. Conventionally, hydrolysis is a mechanism by which drug is released from polymeric materials such as gels (i.e., the drugs are dispersed inside the gel, and they are released slowly as the gel hydrolyzes). However, it is extremely difficult to control the hydrolysis rate of the material, and the amount of water in the body differs depending on the site where the material is implanted, making it difficult to develop a controllable sustained-release form of the drug as long as this conventional method is used. In the development of the next generation of medical gels, a novel approach to the design of materials that feature both tissue adhesion and sustained drug-release is extremely important.
Polysaccharides are often used as main chains for making medical gels (biomaterials) due to the high biocompatibility and large molecular weight. Typical polysaccharides that have been reported as components of biomaterials include hyaluronic acid, chitosan, carrageenan, cellulose, and alginic acid. When applied as biomaterials, hyaluronic acid and chitosan, in particular, have unique and excellent physical properties.
Hyaluronic acid, a linear polysaccharide consisting of alternating linkages of glucuronic acid and N-acetylglucosamine, is found in the extracellular matrix (ECM), synovial fluid of joints, and cartilage1. Hyaluronic acid has anti-inflammatory properties and is both biocompatible and degradable in vivo2. In addition, hyaluronic acid forms hydrogen bonds with a variety of molecules, and occupies a large volume in solution, thus exhibiting excellent water-retention capacity. The high water content of the gel formed by hyaluronic acid leads to suppression of inflammatory reactions and promotion of the angiogenic process and results in excellent anti-adhesion properties. This anti-adhesive effect is a characteristic property of hyaluronic acid gels. To date, various anti-adhesion hyaluronic acid gels have been developed, including a cross-linked structure consisting of formylated hyaluronic acid and adipic acid dihydrazide (ADH)-introduced hyaluronic acid3, and injectable gels composed of methacrylated hyaluronic acid and N-(2-hydroxypropyl) methacrylamide4. On the other hand, hyaluronic acid is degraded by hyaluronidase in vivo and absorbed within 3 days5, making it difficult to maintain the structure and function of the gels with hyaluronic acid alone. Therefore, it is necessary to improve the mechanical properties and degradability of the gels by controlling the cross-link density or by using them with other polymers.
Chitosan is a polysaccharide obtained by deacetylation of chitin, which is found in the shells of crustaceans and the exoskeletons of insects6. Chitin is a polymer made up of repeating units of N-acetyl-ᴅ-glucosamine monomer and is converted to chitosan by deacylation. Chitosan has excellent antibacterial, biodegradable, biocompatible, and cell-adhesive properties7, and because it is an abundant natural resource, it is expected to be applied to a variety of materials. In addition, chitosan is expected to have long-term therapeutic effects when applied to living bodies because of its long degradation time compared to other natural polymers. Chitosan exhibits excellent wound-healing effects by promoting the infiltration of leukocytes into the wound, phagocytosis of foreign substances in living tissues, formation of granulation tissue, and rapid epithelialization. Chitosan also promotes the release of bioactive substances such as prostaglandins, which are important for wound healing, and platelet-derived growth factors at the affected site. Chitosan is the only natural cationic polysaccharide used in the medical field, and its antimicrobial activity and hemostatic activity can be attributed to its cationic nature (i.e., its capacity to interact with anionic cells)8,9. Because it exhibits these excellent characteristics and its excellent biocompatibility, chitosan has also been considered for application in various medical materials. However, in order to actually use chitosan gel medically, it is necessary to overcome the difficulty of gel preparation due to the low water solubility of chitosan. To date, various methods have been investigated to improve water solubility of chitosan including modifying it with other molecules. For example, it has been reported that the introduction of alkyl or carboxymethyl groups can improve the water solubility of chitosan by inhibiting intermolecular interactions of chitosan10,11.
Block copolymers consist of multiple synthetic covalently-linked polymers with different properties. Hydrophilic polymers and hydrophobic polymers have a water-oil relationship and tend to separate from each other. However, within the molecule of a block copolymer obtained by combining hydrophobic and hydrophilic polymers, each polymer cannot be macroscopically separated, and thus a phase-separated structure (microphase-separated structure) appears at the molecular level. The size of microphase-separated structures (cylinder structures, micelle structures, etc.) is limited to the space (tens to hundreds of nm) in which the polymers spread out. In particular, the ability of micelle structures formed in water (polymeric micelles of several tens of nm in diameter) to easily encapsulate hydrophobic compounds such as anticancer drugs has led to active investigation of these structures for their possible medical application12-15.
By incorporating the polymeric micelles as a cross-linked structure inside the polysaccharide material, biomaterials that can freely control the release of drugs and adhere to living tissues can be fabricated (Fig 1). Polysaccharide gels with polymeric micelles incorporated into the internal structure have various properties including the property of: (1) forming quickly, (2) forming only by mixing two kinds of aqueous solutions, and thus easy preparation in operating rooms, (3) low tissue permeability since cross-linkable polymeric micelles are large molecules (molecular weight is about 3-4 million), and (4) high adhesive strength and gel strength.
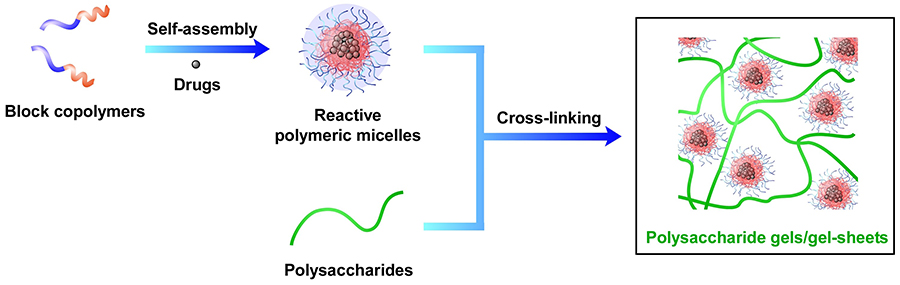
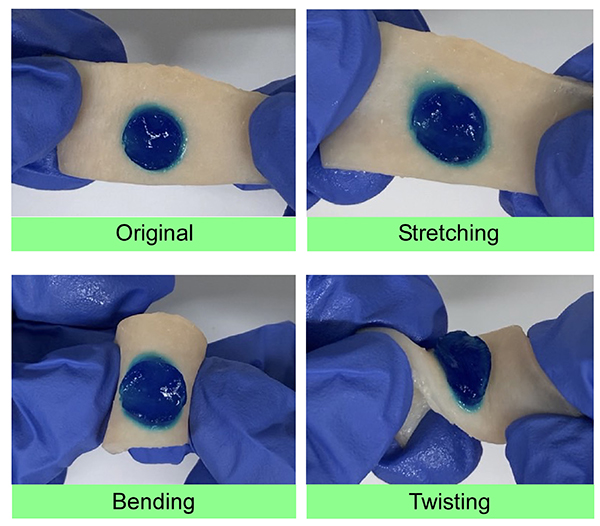
When a surface-formylated polymeric micelle solution is mixed with an aqueous aminated polymer solution, a gel is rapidly formed within a few seconds16-19. For example, gels consisting of polymeric micelles and hyaluronic acid adhere firmly to the surface of biological tissues and show high tissue adhesion and tissue conformability (Fig 2). When a gel forms in an affected area, a short gelation time is a very important property, because if the gelation time were long, the gel would flow out of the affected area before gelation could occur. In addition, the higher the concentration of the two gel components, the higher the proportion of cross-linked structure, and thus the higher the gel strength. When this technology is used in surgery, the viscosity of the two solutions need to be high to avoid their dissipation in the applied affected area. Therefore, it is convenient that the higher the concentration of the two components, the higher the gel strength.
By processing the gel into a sheet, a polysaccharide gel-sheet that adheres to living tissue can be developed20,21. By introducing a synthetic polymer (polyethylene glycol, PEG) into chitosan, which has extremely low water solubility, PEG-modified chitosan (chitosan-g-PEG) with improved water solubility is obtained. By mixing this PEG-modified chitosan with surface-formylated polymeric micelles, a chitosan gel-sheet can be prepared (Fig 3). When this gel sheet was applied to a circular excisional wound formed on the back of a rat, remarkable enhancement of wound healing was observed on day 7, and scar formation was suppressed on day 14. This is presumably due to the fact that the gel-sheet kept the wound in a moist environment, allowing the bioactive substances to move actively, and that the gel provided a good scaffold for epithelialization, thereby promoting healing. In addition, when gel-sheets were prepared using micelles containing a drug (tretinoin) that promotes fibrogenesis, the gel-sheets containing the drug showed higher wound healing efficacy than the gel-sheets that did not contain the drug.

Although various polysaccharide gels are easily developed, it has been difficult to develop polysaccharide gels that have both tissue adhesion and sustained drug release properties. Polysaccharide gels and gel-sheets obtained by the approach presented in this article (fusion of polysaccharides and synthetic polymers) have exhibited high tissue adhesion, sustained drug-release, and wound healing properties. The results obtained to date are expected to lead to the realization of various next-generation medical treatments such as “postoperative therapy” in which a gel encapsulating a factor that promotes wound healing is implanted in the surgical site during surgery and continues to provide treatment after surgery, and “implantable drug-release devices” in which a liquid gel precursor material is injected subcutaneously and gelatinized inside the body. It is also possible to fabricate even smaller polysaccharide gels (polysaccharide gel particles) by using an approach different from that described in this article, and it has been shown that these polysaccharide gel particles possess characteristic drug release behavior22-24. Polysaccharide gels processed into various forms (gels, gel sheets, and gel microparticles) are expected to have a wide range of medical applications in the future.