
Sei Motouchi
Department of Applied Biological Science, Faculty of Science and Technology, Tokyo University of Science
Dr. Motouchi completed his doctoral course at the Department of Applied Biological Science, Graduate School of Science and Technology, Tokyo University of Science, obtaining a degree of doctor of science in March 2025. Since 2025, he has been in his current position as a Japan Society for the Promotion of Science Special Researcher PD (status changed from DC2).

Masahiro Nakajima
Department of Applied Biological Science, Faculty of Science and Technology, Tokyo University of Science Associate Professor
Dr. Nakajima completed his doctoral course at the Department of Biotechnology, Graduate School of Agricultural and Life Sciences, The University of Tokyo in 2006. The same year, he was appointed a NARO Special Researcher at the National Food Research Institute, National Agriculture and Food Research Organization. He then moved to the Iwate Biotechnology Research Center in 2010, where he served as a Researcher. He was appointed Assistant Professor at the Department of Applied Biological Science, Faculty of Science and Technology, Tokyo University of Science in 2012, a Lecturer in 2017, and Associate Professor in 2020.
Glycans related to β-1,2-glucans, acting as a host immune evasion factor, are synthesized by various bacteria. In particular, osmoregulated periplasmic glucans synthesized by root nodule bacteria and plant pathogenic bacteria are essential for their symbiosis and pathogenicity, and it is expected that new agrochemicals and pharmaceuticals will be developed as a result of the synthesis and acceptance of these glycans as control targets. However, many of the synthases and receptors that can serve as targets have not been identified. A novel family of OPG synthases (GH186), which has been discovered by functional and structural analyses by the authors, was identified as being key to the synthesis of two of the three major natural OPG. This paper reports a unique substrate recognition mechanism suggested by these analyses and the existence of new molecular mechanisms for glycan synthesis and degradation reactions.
While β-1,2-glucans may be considered rare in terms of natural abundance, there has recently been increasing evidence for their important roles in plant symbiotic microorganisms and biological interactions between animals/plants and pathogenic bacteria1. These functional glycans are generically called osmoregulated periplasmic glucans (OPG) and reportedly synthesized by a wide variety of bacteria, including the model organism, Escherichia coli1.
The OPG related to β-1,2-glucans are structurally divided into three types as follows:
1. Cyclic β-1,2-glucan (CβG): Has a cyclic structure with β-1,2-glucan as the backbone
2. OPG1: A β-1,2-glucooligosaccharide having a β-1,6-glucose side chain.
3. OPG2: A cyclic β-1,2-glucan containing one α-1,6-glucosidic linkage.
CβG, in particular, was historically the earliest to be studied. In 1940, its existence as a polysaccharide secreted from Rhizobium radiobacter (formerly known as Agrobacterium tumefaciens, a bacterial species that forms nodules on plant roots)2 was suggested and its identity as CβG was later confirmed3. Furthermore, “cyclic β-1,2-glucan synthase (CGS)” was identified as an enzyme producing CβG4. A previous study identified this enzyme as a membrane protein consisting of three carbohydrate-related enzyme domains: glycosyltransferase (GT) family 84 domain, glycoside hydrolase (GH) family 94 domain, and GH189 domain5.
OPG1 and OPG2 are synthesized by Escherichia coli and the phytopathogenic bacterium Xanthomonas campestris pv. campestris (Xcc), respectively1. It is conjectured that the former serves as a ligand for a two-component bacterial control system known as the RcsC phosphorelay system and is involved in the control1,6. RcsC is recognized as controlling the expression of genes encoding flagellar structural components and plant cell wall degrading enzymes. In fact, consistently, bacterial mutations for lack of OPG1 synthesis have been reported to cause flagella formation failures and the loss of phytopathogenicity (e.g., Dickeya dadantii) in various bacteria species1,6. The latter, OPG2, has also been identified as an essential glycan for phytopathogenicity7. In both OPG, however, the synthetic pathway remained almost unknown biochemically. Therefore, there were no clues for drug discovery targeting such a synthase. Aiming to find applications, including agrochemical development targeting OPG-related enzymes, and to deepen our understanding of the influence of OPG on the host, the authors attempted to identify an unknown OPG synthase and elucidate its function and structure in detail.
OPG1 has been suggested to have its β-1,2-glucan backbone synthesized by a membrane-associated GT family 2 enzyme, called OpgH; however, the identity of the proteins involved in the addition of β-1,6-glucose side chains and the control of backbone length remains unknown1. In this situation, focusing on the gene products of opgG, a gene presumably involved in OPG1 synthesis judging from available genetic findings, and its paralogue opgD (EcOpgG and EcOpgD, respectively), functional analyses were performed using recombinant proteins produced with Escherichia coli as the host.
When tested for their substrate specificity for polysaccharides, both proteins were found to be β-1,2-glucanases that specifically hydrolyze β-1,2-glucans as endo-type hydrolases to produce β-1,2-glucooligosaccharides8. Thus, both proteins were shown to be novel enzymes regulating OPG1 chain length. These enzymes were newly registered as the GH186 family because they had no homologs in the existing GH family, and no steric structural similarity to any existing GH was identified. We also experimentally examined the mutarotation of the reaction product, thus demonstrating that EcOpgD relies on an anomer-inverting mechanism8.
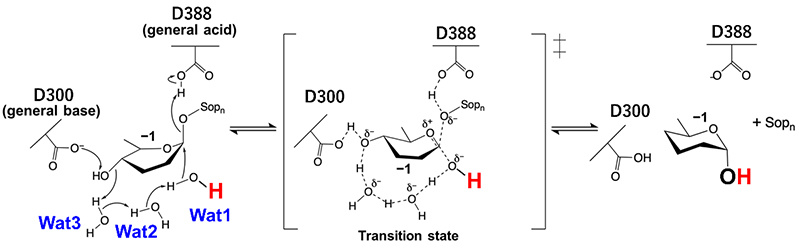
Furthermore, we identified two acidic amino acid residues as the catalytic residues based on an X-ray crystallographic image of the Michaelis complex structure of EcOpgD with β-1,2-glucan (Figure 1), and proposed the following unique reaction mechanism (Figure 2)8:
• Hydrolysis proceeds as the general acid residue donates the proton necessary for glycosidic linkage cleavage, with the general base residue pulling out the proton of the nucleophilic water that nucleophilically attacks the anomeric carbon atom of the glucose unit at the cleavage site via two water molecules and one hydroxy group of the substrate (blue dashed lines in Figure 1 right panel, Figure 2).
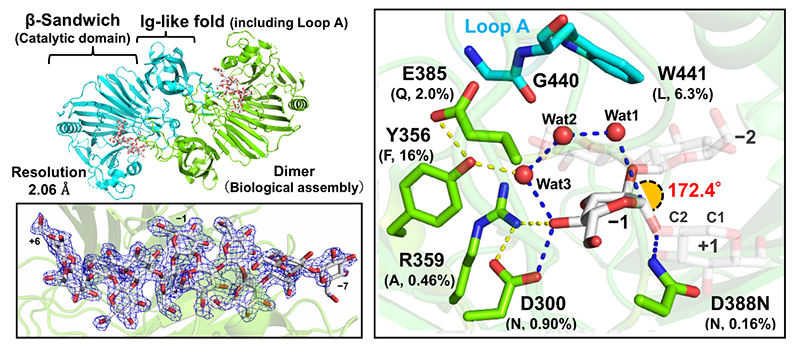
On the general base catalytic residue side, in particular, a proton relay of water molecules (Grotthuss proton relay, GPR) is characteristic. This GPR is generally recognized as occurring randomly in aqueous solvent irrespective of the enzymatic reaction; therefore, control of the randomness of GPR occurrence is considered to be directly related to the efficiency of the hydrolysis. In EcOpgD, a loop region named “Loop A” isolated the GPR pathway from the solvent (Figure 3 left panel), and this is consistent with the finding that EcOpgD exhibits a high reaction efficiency (specific activity, 13.9 U/mg under 8 mg/mL β-1,2-glucan as a substrate concentration)8. In EcOpgG, on the other hand, the GPR pathway is partially accessible to the solvent (Figure 3 right panel); in fact, the specific activity of EcOpgG is only approximately 100-fold less than that of EcOpgD at the same substrate concentration8.
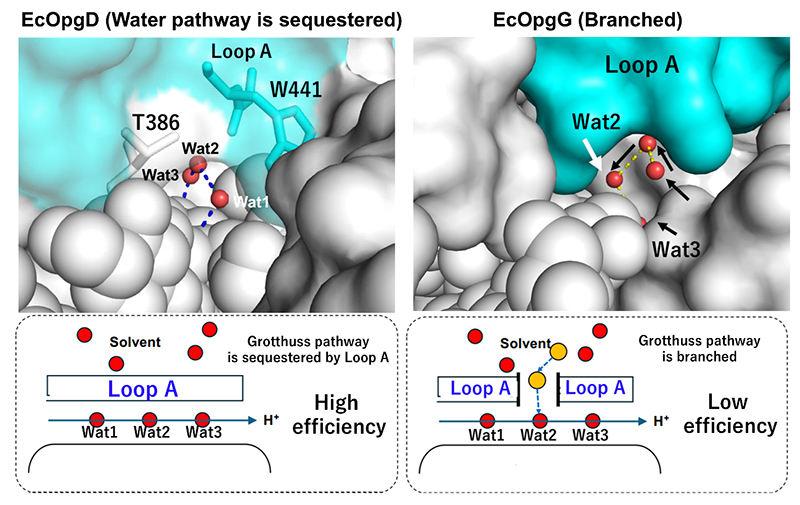
While the structural difference in above Loop A accounts for the difference in hydrolysis efficiency, this Loop A is involved not only in the GPR pathway but also in nucleophile retention. However, among the members of the GH186 family, the Loop As of EcOpgD and EcOpgG are conserved in a restricted part of the phylogenetic tree8. Because the majority of the homologs have a shorter Loop A than that of EcOpgD, it was conjectured that reaction mechanisms might differ depending on the homolog. Regarding OPG synthesis by the phytopathogenic bacterium Xcc, there is no information on OPG1 synthesis by Xcc, while Xcc is reported to synthesize OPG21. Nevertheless, Xcc was found to have a GH186 homolog. Hence, the authors considered the possibility of synthesis of cyclic β-1,2-glucans by a GH186 homolog from Xcc (XccOpgD) and proceeded to perform a functional analysis of XccOpgD.
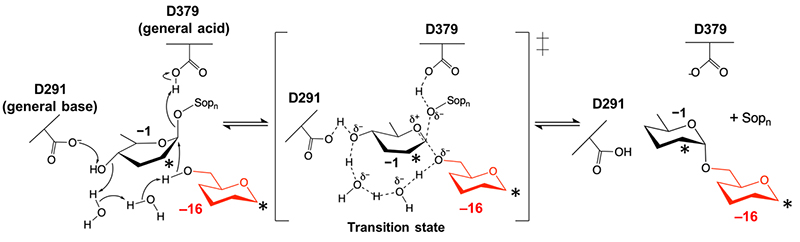
After a reaction was carried out with β-1,2-glucan as the substrate, using purified recombinant XccOpgD produced with E. coli as a host, a product considered to have a specific degree of polymerization was detected. Analysis of this product by mass analysis and NMR identified XccOpgD as an enzyme that specifically synthesizes OPG2 with a degree of polymerization of 1610. Hence, XccOpgD was considered to catalyze transglycosylation by cleaving β-1,2-linkage and transferring a β-1,2-linked glycosyl group in a β-1,2-glucan molecule intramolecularly via an α-1,6-linkage. However, transglycosylation is generally mediated by anomer-retaining GH, whereas the anomer-inverting transglycosylation, which was suggested to be associated with XccOpgD, is a special reaction reported to occur only in connection with some GH91 enzymes11. In addition, no example has suggested a reaction mechanism structurally. Hence, the authors performed X-ray crystallography to evidence the reaction mechanism structurally.
A Michaelis complex structure was successfully acquired at a resolution of 2.25 Å after soaking β-1,2-glucan as a substrate into a ligand-free crystal prepared using XccOpgD D379N (Figure 4). This structure lacked the electron density of a nucleophilic water observed in EcOpgD, instead having a 6-hydroxy group of glucose unit at subsite −16 in the vicinity of the anomeric position at subsite −1 (Figure 4, right panel)10. This configuration strongly suggested the formation of an α-1,6-glycosidic linkage between the two positions (subsites), representing the first case of structurally demonstrating the mechanism for anomer-inverting transglycosylation reaction (Figure 5)10.
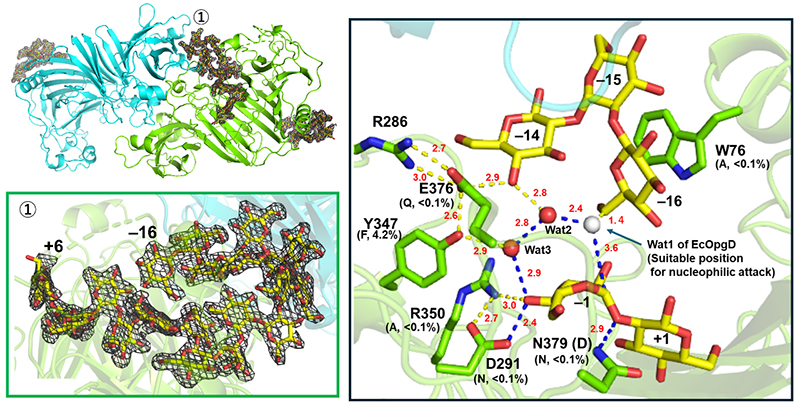
The discovery of the synthase and the elucidation of its detailed reaction mechanism in the present study will accelerate advances in basic research and practical applications in the relatively unexplored field of β-1,2-glucans. For example, the authors envision advances beyond those described in the present study as follows:
Creation of a novel agrochemical: A novel pathogenicity inhibitor, an inhibitor of GH186, will be designed based on the identified Michaelis complex structure.
Elucidation of the bioactive mechanism of OPG in nature: With the discovery of the synthase enabling the mass synthesis of OPG2 by an enzymatic approach, we can now elucidate the mechanism of its action on plants and search for proteins responsible for mutual bacterial communication via OPG2.
Insights into bacterial phenotypes: It may be possible in the future to elucidate, microbiologically, the survival strategies of bacteria with poorly defined life cycles from distribution of genes encoding OPG2 synthase.
Accordingly, β-1,2-glucans, as glycans of suggested importance but with many unknown aspects, are expected to lead to research that spans multiple fields including glycoscience, microbiology, plant pathology, and drug discovery. Further academia-industry collaboration is awaited.