
Junko Nio-Kobayashi
Laboratory of Histology and Cytology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University, Sapporo, Japan. Ph.D, Veterinary Medicine
Junko Nio-Kobayashi graduated from Faculty of Veterinary Medicine, Hokkaido University. She earned her Ph.D. degree from Hokkaido University, Graduate School of Veterinary Medicine, and was appointed Assistant Professor at the Laboratory of Histology and Cytology, Faculty of Medicine and Graduate School of Medicine, Hokkaido University in 2006. She has been engaged in the analysis of galectin-expressing cells in various organs under the guidance of Prof. Toshihiko Iwanaga, who specializes in histology. For two years from 2010, she conducted research on galectins in human corpus luteum and Fallopian tubes in Prof. W. Colin Duncan’s laboratory in the University of Edinburgh, UK, as a JSPS Postdoctoral Fellow for Research Abroad. After returning to Hokkaido University, she became a lecturer in 2018.

Tadasu Urashima
Obihiro University of Agriculture & Veterinary Medicine, Obihiro, Japan. Ph.D., Agriculture.
After graduation from Tohoku University (Doctor of Agriculture) in 1986, he started his professional career by studying milk oligosaccharides at Obihiro University. In 1991, he studied glycosyltransferase activity in lactating mammary glands of the tammar wallaby (marsupial) under Dr. Michael Messer in the Department of Biochemistry, the University of Sydney. Then he completed a comprehensive study of milk oligosaccharides of monotremes, marsupials, and several species of eutherians with Dr. Messer. He is interested in how the present milk components have been acquired during the evolution of mammals, especially how the acquisition of α-lactalbumin, a milk protein, has resulted in the appearance of milk oligosaccharides and lactose, and caused their biological significance to change during evolution. Since 2003, he has been a full professor at Obihiro University. At present he is president of the Japanese Society of Dairy Science and a board member of the Japanese Society of Carbohydrate Research (JSCR) and Japanese Consortium for Glycoscience and Glycotechnology (JCGG).

Jun Hirabayashi
Tokai National Higher Education and Research System, Nagoya University, Japan. Ph.D, Science.
After graduated from Tohoku University (Master of Science), he started his professional carrier at Teikyo University under Prof. Kenichi Kasai for the investigation of animal lectins. On the occasion of GlycoXV (Tokyo, 1999), he proposed the concept glycome; for this realization, he moved to National Institute of Advanced Industrial Science and Technology (AIST, Tsukuba) in 2002, and was involved in a series of national projects for glycan engineering, while he was a deputy director in Research Center for Medical Glycoscience (2006~), prime senior researcher of Research Center for Stem Cell Engineering (2012~). Now, he is a designated professor in Institute for Glyco-core Research (iGCORE), Tokai National Higher Education and Research System, Nagoya University, while being a vice president of the Japanese Society of Carbohydrate Research (JSCR) and Japanese Consortium for Glycoscience and Glycotechnology. (JCGG). He is also a visiting professor of Kagawa University (2003~) and Yokohama City University (2019~).

Sachiko Sato
Research Centre for Infectious Diseases, Faculty of Medicine, Laval University, Quebec City, Canada. Ph.D, Pharmaceutical science
Sachiko Sato graduated from Faculty of Pharmaceutical Science, Chiba University. She joined as postgraduate student in the laboratory of Dr. Akira Kobata, the Institute of Medical Science, the University of Tokyo, Japan in 1987. She also worked in the laboratory of Dr. R. Colin Hughes, MRC: National Institute for Medical Research in London, UK, where she first encountered a cytosolic mammalian lectin, now called galectin-3. She obtained her Ph. D. from the University of Tokyo in 1994. As postdoctoral fellow in the laboratory of Dr. Ron Kopito, Stanford University, she was involved in the work on cystic fibrosis. She became principal investigator of the laboratory of glycobiology in Research Center for Infectious Diseases, and assistant professor of the Faculty of Medicine, Laval University, Quebec, Canada in 1999 and is full professor since 2010. She is also director of the Bioimaging platform since 2003.
The binding affinity of lectins for glycans is generally displayed at the µM level; however, milk contains more than 10 mM oligosaccharides and 100 mM lactose. Such an astonishingly high concentration of glycans present in milk could inhibit the binding of most endogenous lectins to their glycan ligands. Then, many questions naturally arise, for example: what is the biological significance of the inhibitory activity? How high are the milk oligosaccharide concentrations in the digestive tract, and what is the pharmacokinetics of milk oligosaccharides in the body?
In this spin-off version, we try to present our guesses or “daydreams” – our extended speculation – and discuss about how milk oligosaccharides are used once they enter the body, what roles they may play, and what their relationship may be to the function of galectins, which are abundant in the digestive tract. In addition, we reconsider the reason why mammals have acquired the ability to biosynthesize lactose and use it as a nutrient source for babies, which is said to be the “key to the evolutionary success of mammals”.
As discussed in the main chapter “Milk oligosaccharides and Galectins” by Urashima and Hirabayashi (Glycoforum. 2021 Vol.24 (2), A3) 1, milk contains high concentrations of lactose and oligosaccharides. Lactose in milk is digested into galactose and glucose by lactase secreted from absorptive epithelial cells in the small intestine, and absorbed as an energy source. In contrast, enzymes that digest milk oligosaccharides, especially human milk oligosaccharides with complex composition, have not been identified at least in the human gut epithelia2,3. A majority of milk oligosaccharides pass through the small intestine without being digested, and reach the large intestine where they are used by enterobacteria.
In breast-fed infants, Bifidobacterium is the predominant intestinal enterobacteria. As prebiotics, milk oligosaccharides are actively utilized by beneficial colonic bacteria such as Bifidobacterium and Lactobacillus, promoting their growth and colonization. Katayama at Kyoto University and Kitaoka at Niigata University reported that Bifidobacterium possesses the enzymes that digest milk oligosaccharides (Note 1). The amount of milk oligosaccharides and their metabolites in feces of breast-fed infants decreases in association with intestinal colonization of Bifidobacterium4, reflecting an increase in oligosaccharide utilization by these intestinal colonizers. On the other hand, milk oligosaccharides are also known to have an inhibitory effect on the infection of harmful bacteria and viruses that adhere to intestinal epithelium via sialic acids (Note 2).
Importantly, as discussed below, certain amounts of milk oligosaccharides enter the circulation and are excreted in urine.
Note 1) The predominant enterobacteria in infants before weaning are Bifidobacterium breve, B. bifidum, B. longum subsp. infantis, B. kashiwanohense. These species all possess enzymes that metabolize milk oligosaccharides5,6 After weaning, these Bifidobacterium species are replaced by adult-type Bifidobacterium species (B. adolescentis, B. longum subsp. longum, B. catenulatum). In addition, Bacteroides and Clostridium species take the place of Bifidobacterium as the predominant enterobacteria, and participate in the digestion of dietary fibers and mucins secreted from gut epithelia in adults. Acetic acid, lactate, and butyrate secreted from these enterobacteria optimize ion secretion from gut epithelia and also are used as energy sources by enterocytes. Bifidobacterium and Clostridium produce acetic acid and butyrate, respectively7. In the gut of neonatal mice, acetic acid produced by Bifidobacterium plays an important role in establishing the normal peristaltic movement of the gut, and non-neuronal acetylcholine is involved in this action8. Please refer to another Glycoforum series “Human Milk Oligosaccharides” for details of the relationship between milk oligosaccharides and enterobacteria.
Note 2) Bacteria and viruses whose binding to and invasion of host cells are inhibited by milk oligosaccharides are as follows: Campylobacter jejuni, Entamoeba histolytica (protozoa), Group B Streptococcus, Norovirus, and Rotavirus9. These organisms cause infectious gastroenteritis and meningitis in infants. As most of them enter cells by recognizing cell surface sialic acids, milk oligosaccharides containing sialic acids block the entry of these infectious microorganisms. Sialic acids residues are easily released under acidic conditions. Notably, in contrast to the fluid content of the adult stomach (pH 1.5-2), that of the infant stomach is much less acidic (pH 6-8). This weaker acidity is likely linked to the fact that the gastric mucosae is still under development. Thus, it is not likely that sialic acids are chemically removed from milk oligosaccharides as they pass through the infant stomach.
The digestive tract is a continuous tube running from the mouth to the anus and includes the esophagus, stomach, and small (duodenum, jejunum, ileum) and large intestines. In the stomach, food is mixed with gastric juice to form porridge and sent to the small intestine. In the duodenum, it is digested by the action of bile from the liver and pancreatic enzymes, and food nutrients are absorbed in the jejunum and ileum. In the large intestine, water and ions are absorbed and the debris is excreted as feces.
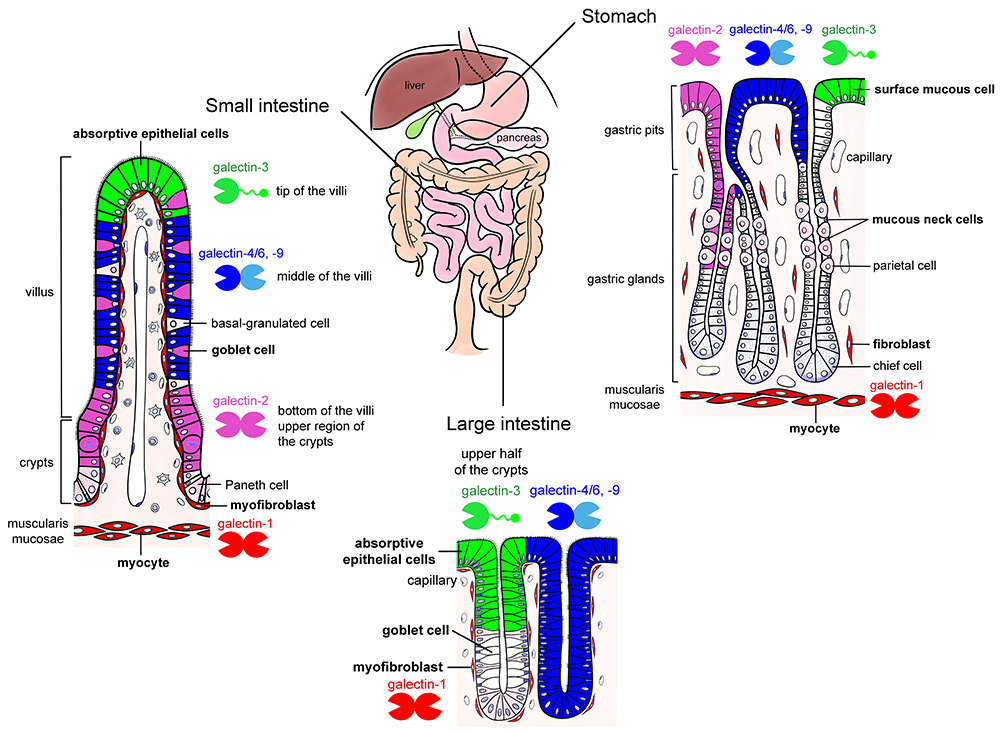
The mucosal epithelium of the gut abundantly expresses galectins. One of the authors (Nio-Kobayashi) reported that at least six galectins (galectin-2, -3, -4/6, -7, and -9) are differentially expressed in a region- and cell-specific manner in the mucosal epithelium of adult mice10 (Fig. 1). While the function of galectins in the gut epithelium remains unclear, they are suggested to be involved in cell differentiation and proliferation, as well as protection from infection (Note 3).
Note 3) Galectin subtypes change in association with the differentiation and maturation of stomach and intestinal epithelial cells that express multiple galectins. For example, in the small intestine, galectin-2 is expressed in the absorptive epithelial cells in the crypt (proliferative zone) and at the base of villi, while galectin-4/6 and -9 are detected midway between the base and tip of the villi, and galectin-3 exists at the tip of villi (Fig. 1). Such distribution suggests an involvement of galectins in the differentiation and maturation of epithelial cells; however, the function of galectins and the characterization of their ligand glycoconjugates remain elusive. The mucus-producing cells (surface mucous cells and mucous neck cells) in the stomach secrete mucin-type glycoproteins, and both cells express galectins (galectin-2, -3, -4/6, and -9). Surface mucin secreted from surface mucous cells contains MUC5AC as a core protein, whereas glandular mucins secreted from mucous neck cells and the pyloric glands contain MUC6. Nakayama at Shinshu University established a A4GnT-knockout mouse that lacks an enzyme to transfer N-acetylglucosamine at α1,4 linkage on the terminal galactose of glandular mucins. In A4GnT-knockout mice, the pyloric mucosa thickens with age and becomes hyperplastic, dysplastic, and malignant11. Since A4GnT deficiency exposes the N-acetyllactosamine structure, which can be recognized by galectins, it is considered that the interaction between galectins and these mucins affects the growth of mucosal epithelium and development of mucosal epithelial cancers (Shigeru Kakuta at Tokyo University; unpublished data). Park et al. at Kinki University have reported that galectin-3 produced by gastric mucosal epithelial cells protects against Helicobacter pylori infection12. In addition, the involvement of galectins in cancer progression and inflammatory bowel disease has been suggested13.
The small intestinal epithelial cells of neonates and infants have different characteristics from those of adults: they actively take up molecules from the lumen. With this active uptake function, it is possible for small intestinal epithelial cells to directly take up huge proteins such as antibodies present in breast milk, without being digested14. Here, we would like to discuss the possible relationship between lactose and milk oligosaccharides derived from breast milk and galectins abundantly expressed in the digestive tract.
As mentioned above, most milk oligosaccharides reach the large intestine without being digested, and are utilized by enterobacteria. However, at least 1-3% of intact milk oligosaccharides is detected in the blood and urine of breast-fed infants (Note 4), suggesting that some oligosaccharides are indeed absorbed within the digestive system. Although the amount is not large relative to the total amount of ingested milk oligosaccharides (0.1% in plasma and 4% in urine compared to the concentration in milk), they likely function in the body15.
Noteworthily, since the original concentration of oligosaccharides in milk is quite high, its concentration in the body reaches more than 100 µM even though only a small percentage is absorbed. At this concentration, inhibition of the binding of most of endogenous lectins to glycans is anticipated. For example, Bode et al. reported that human milk oligosaccharides containing the sialyl-Lewis X structure, a selectin ligand, suppress inflammation by inhibiting selectin-mediated binding of leukocytes to inflammation-stimulated endothelial cells16.
Note 4) Kunz at University of Giessen confirmed that 13C-labeled galactose was found in the milk and urine of breast-fed babies of mothers who are orally given 13C-labeled galactose17. Goehring et al. and Ruhaak et al. also demonstrated that milk oligosaccharides exist in the blood of breast-fed babies18,19.
Lactose found in milk is an antagonist of galectins, and most milk oligosaccharides produced from lactose also interact with galectins20 (Note: galectins cannot bind to oligosaccharides possessing a sialic acid modification at the C6 position of galactose and Lewis structures. For details, refer to the main chapter “Milk Oligosaccharides and Galectin”1.).
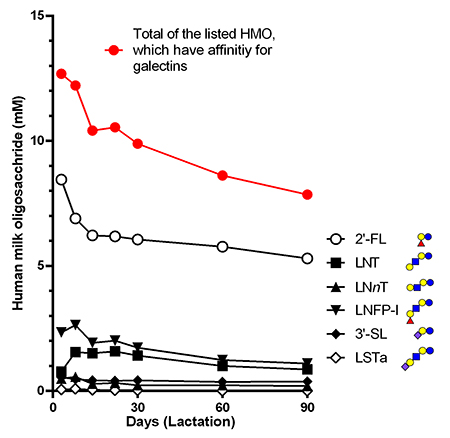
Regarding the oligosaccharides in human milk reported by Urashima et al.1, the molar concentration in milk of those having an affinity for galectins was calculated to be 12 mM (colostrum) and 8 mM (90 days after birth) in total (Fig. 2). The concentration of lactose in milk is 45 g/L (bovine) or 70 g/L (human), which corresponds to molar concentrations as high as 130-200 mM. Considering the dissociation constant of galectin binding to lactose and oligosaccharides (0.01 to 0.1 mM)21, the concentration even when diluted 100-fold is sufficient to inhibit galectin binding.
This implies that lactose and milk oligosaccharides act as “stripping agents” for galectins. However, the biological significance of this “stripping action” has not been studied yet.
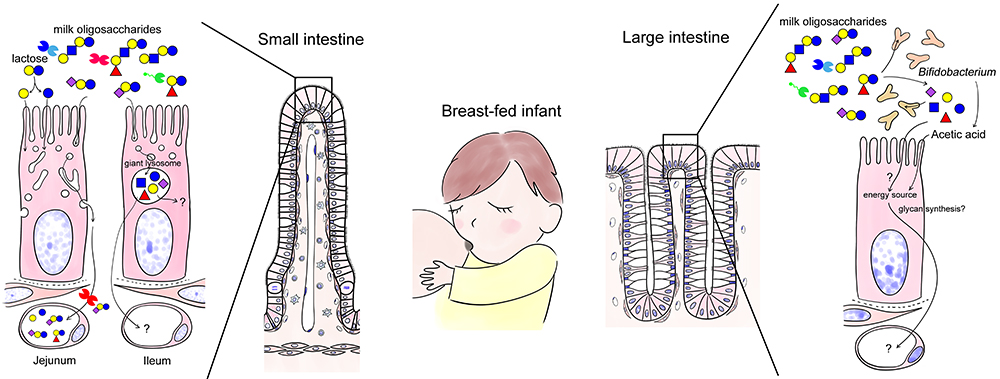
As mentioned above, the epithelial cells of the small intestine in neonates and infants are “phagocytic” and actively take up nutrients and proteins from the lumen while the fate of the incorporated molecules seem to differ in the jejunum and the ileum22. In the jejunum, macromolecules such as antibodies are taken up and carried to the baso-lateral side without being digested, whereas in the ileum, the absorbed substances are collected and digested in giant lysosomes present in supra nuclear region (Fig. 3). Milk oligosaccharides are expected to be taken up by enterocytes and enter the circulation through the same pathway as antibodies (Jejunum; left in Fig. 3).
When human milk oligosaccharides are added to Caco-2 cells, a human colon cancer cell line, neutral human milk oligosaccharides that are not modified with sialic acid are taken up and carried to the basolateral side but not if they are modified23. Most milk oligosaccharides reach the large intestine without being digested. In contrast, 3’-sialyllactose (3’-SL), 6’-SL, and 2’-fucosyllactose (2’FL) are uptaken in the small intestine and enter the circulation in rats24,25.
It is possible that milk oligosaccharides are digested by giant lysosomes in the ileum (Ileum; left in Fig. 3). Jantscher-Krenn et al. administered human milk oligosaccharides to rats and measured their concentrations in each part of the gastrointestinal tract24. The jejunal concentration of 2’-FL, lacto-N-tetraose (LNT), and lacto-N-neotetraose (LNnT) decreased whereas that of lacto-N-fucopentaose I (LNFP-I), LNFP-II, and sialyl lacto-N-tetraose b (LSTb) increased compared to the orally administered concentration. On the other hand, galactooligosaccharides (lactose elongated by artificial transfer of galactose; they are currently being used as prebiotics) are partly digested in the small intestine but do not enter the circulation. Although the pharmacokinetics of milk oligosaccharides may differ between rats and humans, it is likely, depending on their structure, that some milk oligosaccharides are easily absorbed or digested in the small intestine, while others are not.
Several reports suggest that milk oligosaccharides can alter the expression of proteins in the digestive system. For example, administration of milk oligosaccharides increases gastrointestinal mucosal barrier function (tight junctions become stronger) and mucin production26. Necrotizing enterocolitis is a common disease in pre-term ultra-low weight infants. In addition to impaired blood flow to the intestine, bacterial infection causes necrosis of the intestine. Breast milk prevents necrotizing enterocolitis, probably through the action of human milk oligosaccharides, particularly disialyl-lacto-N-tetraose (DSLNT), that enhance the barrier function of the intestinal epithelium27.
In the analysis using HT-29 cells derived from human colorectal cancer, the expression of galectin-3, -4, -9 increased when the CpG motif, a bacterial antigen, and 2’-FL were treated together28. Although the subtype-specific distribution of galectins in the gastrointestinal epithelium has been established in the embryonic period29, the intensity of each galectin subtype seems to differ between neonatal and adult mice (Nio-Kobayashi, unpublished data). Milk-derived lactose and oligosaccharides are anticipated to inhibit the binding of extracellular galectins to their ligand glycans, and also to induce or suppress the expression of galectins in the gastrointestinal epithelium, while such possibilities have not been previously addressed. Expanding upon this “daydream”, intracellular galectins, which expression is increased by milk oligosaccharides, may contribute to the development of normal gastrointestinal mucosa and to increased resistance to infection, as they are involved in the repair of damaged lysosomal membrane and autophagy30.
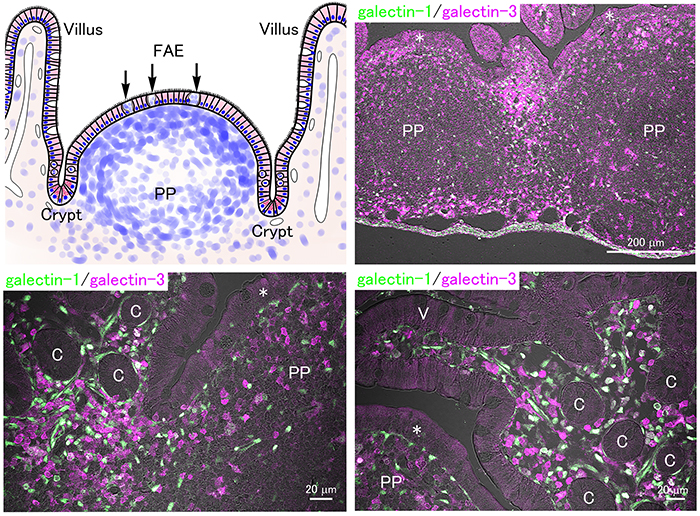
In addition to nutrient absorption from foods, the digestive tract functions as an immune organ containing numerous immune cells. Immune cells in the lamina propria and Peyer’s patches, aggregated lymph nodules in the ileum, play a central role in mucosal immunity. Since the germinal center of the Peyer’s patch in mice develops rapidly after weaning31, the immune function of the offspring may be suppressed and depend on the immunoglobulins from milk produced during lactation. Microfold (M) cells possess phagocytic activity and are distributed within the follicle-associated epithelium where they take up and pass luminal substances to immune cells that are present beneath, activating the immune response.
In the small intestine of adult mice, numerous galectin-expressing immune cells are distributed in the lamina propria and Peyer’s patches (Fig. 4). The concentration of milk oligosaccharides in plasma is not high (0.1% in milk, namely about 0.1 mM) yet potentially high enough to inhibit the extracellular lectin activity of galectins. Further, the local concentration of milk oligosaccharides taken up via epithelial cells and/or M cells in the lamina propria and Peyer’s patches would also inhibit the interaction between galectins and glycans, but such a possibility has never been directly investigated.
Fetal and neonatal immune system is skewed toward Th2 humoral immunity, but is shifted toward Th1 cell-mediated immunity after birth. Bacterial and viral infections in childhood are thought to be important for this transition and to be involved in reducing the risk of allergies in the future. This proposition is known as the hygiene hypothesis32 (Note 5).
It has been reported that milk oligosaccharides in human colostrum shift the cytokine balance in the fetal intestinal mucosa toward a Th1 state33-35.
Note 5) Proposed in 1989 by the British epidemiologist David Strachan, the hygiene hypothesis is the concept that hygiene in infancy affects the development of an individual’s immune system and determines whether an individual is prone to allergies. Accordingly, it was hypothesized that exposure to microorganisms during infancy is necessary for the development of a normal immune system, and inadequate exposure leads to the development of future allergic diseases.
Adult mice lacking galectin-1 or -3, and mice with reduced biosynthesis of glycans that have high affinity for galectin tend to mount higher Th1 responses36-39. Thus, it is conceivable that milk oligosaccharides skew the neonatal immune response towards Th1, possibly by inhibiting galectins.
Most recently, the relationship between the bifidobacterial genes involved in milk oligosaccharide metabolism and the circulating mucosal-specific T cells and other immune cells in infants was extensively analyzed34. The analysis suggests a strong positive correlation between the presence of genes for milk oligosaccharide metabolism in Bifidobacterium and a higher number of immunoregulatory mucosal immune cells such as regulatory T cells as well as the immunoregulatory cytokine, IL-27, which limits Th2 and Th17-type immune responses, skewing the immune balance towards a Th1 and regulatory T cell response.
Administration of Bifidobacterium expressing milk oligosaccharide-utilization genes to infants reduced systemic inflammation, especially Th2 and Th17 immune responses34. Thus, in breast-fed infants, the oligosaccharides likely help the colonization of Bifidobacterium, which skews mucosal immune responsiveness in a Th1 and regulatory immune direction.
Interestingly, based on the experiments using in vitro immune polarization assays with adult peripheral naïve CD4 T cells, this study also showed that indole-3-lactic acids (ILA) produced by Bifidobacterium could induce the transcription of galectin-1 and CXCR3, which is often associated with Th1. They propose that galectin-1 is one of the factors involved in the Bifidobacterium-induced skewing towards Th1 responses34.
However, galectin-1 is known to skew immune responsiveness towards Th2 since galectin-1 induces apoptosis in Th1 and Th17 but not Th2 cells where modification of surface glycans with α2,6-sialylation prevents galectin-1 binding37. Moreover, even if the induction of transcription of galectin-1 in ILA-exposed Th0, Th2, and Th17 cells was observed34, it is expected that milk oligosaccharide present in the digestive tract can inhibit the extracellular lectin activities of galectins. Nonetheless, the exciting explosion of research addressing the functions of milk oligosaccharides in the development of the infant mucosal immune system will further reveal the relationship between milk oligosaccharides, Bifidobacterium, galectin, and immunity conferred by breast feeding in the very near future.
The abundance of milk oligosaccharides in colostrum gradually decreases during the lactation period. The composition of milk oligosaccharides changes during the lactation period: 2’-FL, which binds to galectins, is the first to predominate (20-40% of total milk oligosaccharides) in colostrum; then 6’-SL, which does not bind to galectins, becomes the dominant form at the early stages of lactation; and lastly 3’-SL, which is a galectin ligand, emerges in late lactation40.
Therefore, although the issue is unresolved, it is fully conceivable that milk oligosaccharides directly or indirectly regulate the expression and function of galectins in immune cells and thereby immune balance during lactation.
Galectins are abundant both in epithelial cells and submucosal immune cells in the digestive tract; however, their function is poorly understood. Milk oligosaccharides are involved in the proliferation, differentiation, and functional changes of the gastrointestinal mucosa as well as mucosal immunity. Could galectins be involved in these phenomena? In particular, it is expected that galectins play an important role in the development of gastrointestinal mucosa, the optimization of the intestinal environment during the neonatal period, and the regulation of mucosal immunity. As an aside, it is also worth noting that the concentration of milk oligosaccharides in urine is also very high (4% of the concentration in milk). Since the transitional epithelium in the urinary system expresses galectin-310, the milk oligosaccharides in urine may interact with urinary galectin-3.
We hope that the elucidation of the role played by the abundant breast milk oligosaccharides as “stripping agents” for galectins in the infant body will lead to new insights in the near future.
While on the subject, why does milk contain so much lactose? What is the benefit of producing lactose instead of glucose, which is the most accessible energy source, by changing the substrate specificity of glycosyltransferases (Glycoforum. 2021 Vo. 24 (3), A6) 41? It is said that “the acquisition of lactose is the key to the evolutionary success of mammals”, but why did mammals choose lactose? After discussing this point, we found some issues that could be addressed.
First, if glucose had been the sugar in milk, an environment prone to infection by a variety of bacteria would have been created. Beneficial enterobacteria such as Bifidobacterium prefer lactose and milk oligosaccharides5, and produce acetic acid which suppresses the growth of harmful bacteria such as Escherichia coli and Clostridium perfringens. Lactose and milk oligosaccharides may play a role in establishing an appropriate (beneficial) intestinal environment.
Second, it can be pointed out that a high level of glucose becomes “harmful” to cells. When blood glucose concentration is high, a glycation reaction occurs and advanced glycation end products are produced. Notably, the glycation reactivity of glucose is significantly reduced when glucose is modified with other sugars such as galactose (Note 6). Converting glucose to lactose eliminates the risk of glucose toxicity, even at concentrations as high as a few percent.
Note 6) Shinohara et al. compared the glycation (Maillard reaction) activity of glucose with that of other monosaccharides such as galactose and disaccharides in which glucose was modified by various binding patterns42. The glycation activity of galactose was higher than that of glucose, and shown to be increased by α or β1,6-modified glucose. On the other hand, glucoses modified by forming α/β1,2, α/β1,3, and α/β1,4 bonds have relatively low risk of glycation. Since the glycation activity of lactose with the β1,4 bond is lower than that of glucose43, lactose may be said to be a stable (less harmful) sugar.
Third, as mentioned elsewhere, lactose and milk oligosaccharides can also deliver a large amount of sugar without increasing the osmotic pressure.
Finally, why is galactose chosen as a partner to modify glucose in the first place? It may be to supply galactose, which is essential for the synthesis of glycoproteins, proteoglycans, and glycolipids, required for rapid growth together with glucose. Lactose in milk is digested by lactase to glucose and galactose in the small intestinal epithelium. These absorbed sugars are transported through the portal vein to the liver and metabolized (Note 7). However, some may be used for glycan biosynthesis in the gastrointestinal epithelium, although such possibility has not been confirmed (Fig. 3).
Note 7) Newborns lacking genes to metabolize galactose such as galactose-1-phosphate uridyltransferase, galactokinase, and galactose epimerase (Leloir pathway) in the liver, or with abnormal portal vein blood flow, suffer from galactosemia causing vomiting, jaundice, diarrhea, and intellectual disability in infancy.
It seems that the acquisition of the biosynthetic pathways for lactose and milk oligosaccharides was coincidental in biological evolution, but there is no doubt that the acquisition worked in favor of selection41. Living organisms are not completely rational creatures, and they will acquire advantageous traits through a process of trial and error. This is also a kind of “wobbling” of life.
AcknowledgementsAuthors thank Dr. Yasuo Shinohara (Kinjo Gakuin University), and Dr. Takaji Yajima and Dr. Masako Yajima (both retired; both former Meiji Dairies Research Chairs, Creative Research Institution, Hokkaido University) for providing useful information and hints for discussions in the preparation of this manuscript.
We would like to dedicate this forum to Prof. Hans-Joachim Gabius, who had kept challenging to address unsolved questions about galectins.